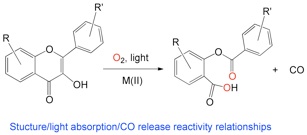
Photoinduced CO-release reactivity of flavonols

M(II)/O2 promoted oxidative aliphatic carbon-carbon bond reactivity
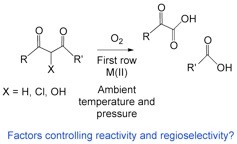
The selective oxidative cleavage of aliphatic carbon-carbon bonds is an important area of investigation due to the role such reactions play in biological systems, as well as the potential use of such reactions in synthetic organic chemistry. Our research is directed at elucidating the chemical factors that influence reactions of this type involving first row divalent metal ions and dioxygen.
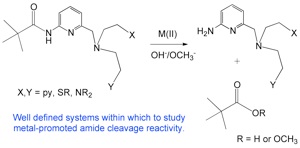
Metal ions play important roles in catalysis in biological systems, with one of the most abundant metal ions being Zn(II). By studying chemical reactions of relevance to those catalyzed by zinc enzymes, our goal is to provide detailed chemical insight that may assist in the development of new therapeutics and contribute toward understanding metal ion toxicity (upon replacement of Zn(II)).
Zinc-promoted reactivity in biological systems; relationships to therapeutic targets and metal ion toxicity
Carbon monoxide is generally thought of a toxic small molecule. However, recent studies indicate that a low concentration, CO is an important signaling molecule in humans and is known to dilate blood vessels and have anti-inflammatory effects. Based on these properties, an area of emerging research is the development of CO-releasing molecules for the controlled delivery of a specific amount of CO. We are contributing to this area through studies of the photoinduced CO-release reactivity of flavonols.
Want to know more, check out our publications page.